
CRISPR gene editing has made a major breakthrough. Intelligent therapeutics and regeneron jointly announced that the in vivo gene editing therapy ntla-2001 jointly developed by them not only significantly reduces the level of pathogenic proteins in patients after one treatment, but also shows good safety.
Professor Jennifer doudna, the co-founder of intellia therapeutics and a pioneer in the field of CRISPR gene editing and the winner of the 2020 Nobel Prize in chemistry, said that CRISPR gene editing took less than 10 years from the initial basic scientific discovery to transformation into clinical research. In her memory, this is one of the fastest transformation technologies.
Where will CRISPR gene editing go in the future? Now let's take a look at the insights shared by Professor Jennifer doudna in the recent interview and the layout of intellia.

Jennifer Doudna professor
Delivery of gene editing systems remains a major challenge to be addressed
Professor Jennifer doudna said that in clinical drug development, how to deliver CRISPR gene editing system to the tissues or cells in the body that want to be targeted is still a challenge that the industry needs to solve.
Intellia currently uses a delivery technology platform based on lipid nanoparticles (LNP). It shows the ability to effectively deliver CRISPR gene editing system to the liver. At the same time, it has many other advantages, including low immunogenicity, the possibility of repeated administration, the ability to be degraded by the body, and so on.
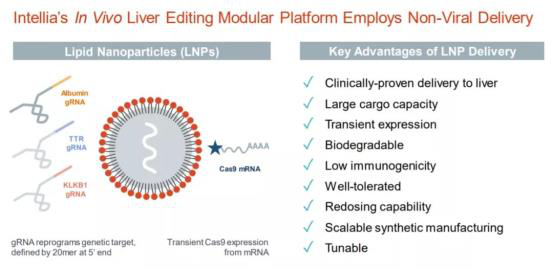
Introduction of liver targeted lipid nanoparticles delivery platform
The results of clinical trials published last weekend showed that ntla-2001, developed using this delivery technology, reduced the level of transthyroxine protein (TTR) in the serum of patients with transthyroxine amyloidosis (attr) by an average of 87%, showing a good gene editing effect.
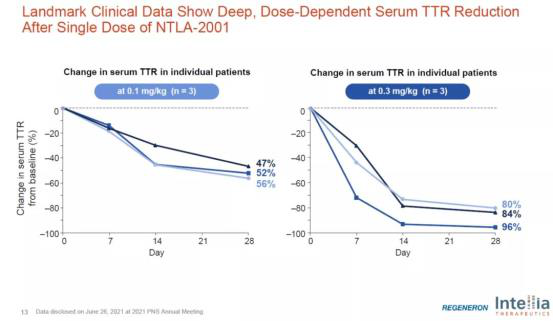
Preliminary clinical results of ntla-2001 reducing TTR level
Using this delivery technology, intellia has been developing in vivo gene editing therapies for the treatment of hereditary angioedema (HAE). It knocks out klkb1 gene in the liver and reduces kallikrein activity, aiming to significantly reduce the frequency of disease attack in patients through one treatment. In the non-human primate (NHP) model, one treatment can reduce kallikrein to a level that affects the frequency of disease attack and maintain it for one year. The company plans to register the first clinical trial patient this year.
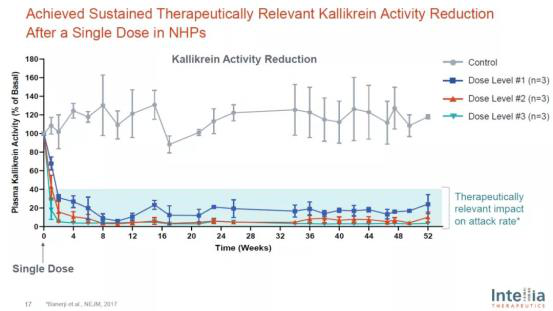
In vivo gene editing therapy for hae significantly reduces kallikrein activity in non-human primate models
Using this delivery technology, intellia can not only accurately knock out genes with abnormal functions in the liver, but also accurately implant genes with normal functions. The company's ongoing treatment of alpha-1 antitrypsin deficiency (AATD) is divided into two steps. First, the gene editing therapy delivered by LNP is used to knock out the gene expressing the mutant protein, and then with the help of the gene editing system delivered by LNP, the DNA is accurately cut in the genome of liver cells to assist in the insertion of normal genes delivered by adeno-associated virus (AAV).
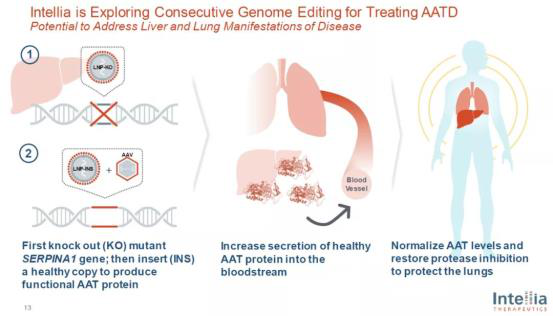
Through gene editing, the gene expressing normal protein can be accurately introduced after the mutant gene is knocked out
In non-human primate models, this treatment has been able to bring normal human antitrypsin expression levels (see figure below).
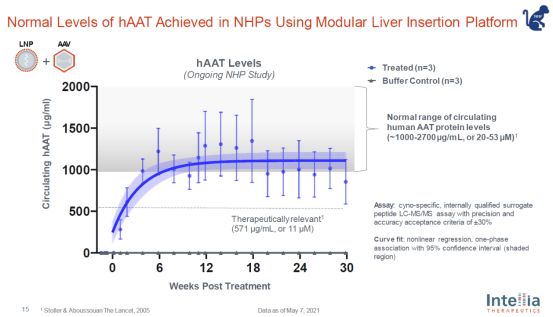
Targeting CRISPR gene editing system to the liver is relatively easy to achieve, because "in the body, the liver is originally an organ that absorbs a large number of molecules", Professor doudna said. However, it is not so easy to deliver gene editing systems to other tissues and organs. "Editing brain, heart or muscle cells has great medical potential, but at present, we have no really effective tools to introduce editing systems into these cells."
Intellia has also recently made progress in developing delivery systems that target tissues other than the liver. In March this year, the company announced the successful in vivo gene editing of hematopoietic stem cells and progenitor cells in mouse models using bone marrow targeted LNPS, and the level of gene editing is expected to achieve a functional cure in patients with sickle cell anemia.
Professor doudna said that using different viral vectors or viroid particle technologies, researchers have made some progress in targeting crisrp systems to tissues other than the liver, such as the brain, heart and muscle. "I am very excited about the innovations that will appear in this area in the next few years." She said.
Main directions of in vitro gene editing
At present, most applications of CRISPR gene editing are still in vitro gene editing. This strategy collects cells from patients, performs gene editing and modification in vitro, and then transfuses them back into patients. Sickle cell anemia is particularly suitable for CRISPR targeting, because it is caused by single gene mutations, and hematopoietic stem cells can be collected, edited, and then transfused back into the patient, Professor doudna said. Otq923/hix763, jointly developed by intellia and Novartis, is a cell therapy that uses gene editing to introduce genes expressing fetal hemoglobin into patients' hematopoietic stem cells. At present, phase 1/2 clinical trials have been launched.
Another major research direction of in vitro gene editing is to create cell therapies for cancer or autoimmune diseases through gene editing systems. This is one of the main development directions of intellia. By using crispr-cas9 gene editing, the company's cell engineering technology can not only carry out multi-step genetic engineering transformation on a variety of cells, but also maintain the health of cells and reduce the occurrence of unnecessary gene mutations.
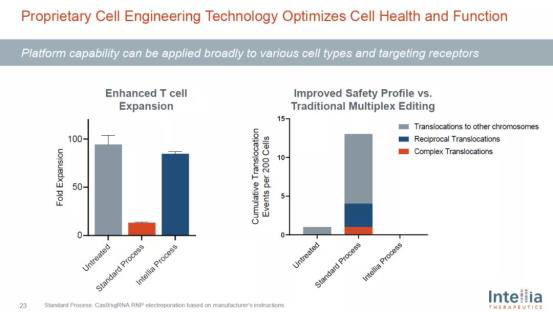
Intellia's cell engineering technology platform can optimize the health and function of cells
Ntla-5001 is a potential "best in class" T-cell therapy targeting WT1 for the treatment of acute myeloid leukemia (AML). WT1 is an antigen overexpressed in more than 90% of AML mother cells. Ntla-5001 uses gene editing technology to introduce high affinity TCR targeting WT1 while removing the innate TCR of T cells.
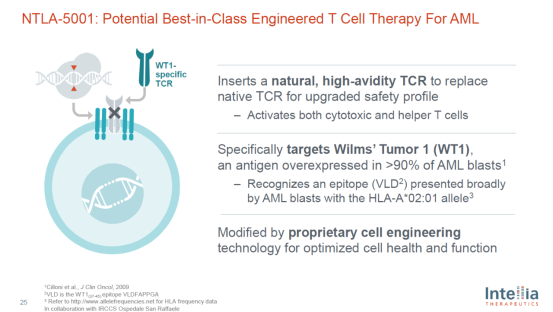
Introduction to ntla-5001
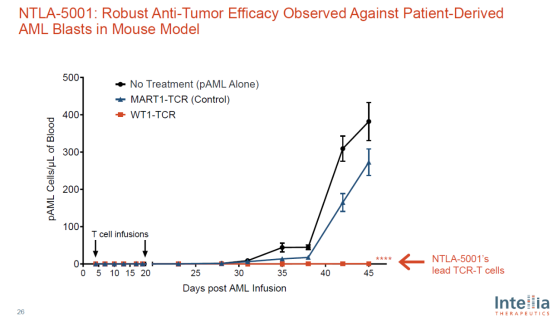
In mouse experiments, this treatment under development has shown strong anticancer activity (see the figure below). It is expected that the company will submit an ind application this year.
The future of CRISPR gene editing is not just medical treatment
Professor doudna pointed out in the interview that with the progress of CRISPR gene editing technology, it can also affect industries outside the medical and health field, in which agriculture may be the first to benefit.
Traditional hybridization methods may take months to years, while CRISPR technology can manipulate specific genes "without changing other places". "This allows people to do many things to address the challenges posed by climate change, such as dealing with drought conditions and introducing pest protection features into plants." She said.
reference material:
[1] Intellia and Regeneron Announce Landmark Clinical Data Showing Deep Reduction in Disease-Causing Protein After Single Infusion of NTLA-2001, an Investigational CRISPR Therapy for Transthyretin (ATTR) Amyloidosis. Retrieved July 1, 2021, from https://ir.intelliatx.com/news-releases/news-release-details/intellia-and-regeneron-announce-landmark-clinical-data-showing
[2] Intellia Therapeutics Announces Pricing of Public Offering of Common Stock. Retrieved July 1, 2021, from https://ir.intelliatx.com/news-releases/news-release-details/intellia-therapeutics-announces-pricing-public-offering-common-2
[3] How CRISPR gene editing will treat diseases in future: Nobel-winning Intellia co-founder Jennifer Doudna. Retrieved July 1, 2021, from https://www.cnbc.com/2021/06/30/how-crispr-gene-editing-will-treat-disease-intellia-founder-doudna-.html
[4] Intellia Corporate Overview. Retrieved July 1, 2021, from https://ir.intelliatx.com/static-files/a57908a6-aaca-404d-9072-32471cd3d41e
Note: This article aims to introduce the research progress of medical health, not to recommend treatment schemes. If you need guidance on the treatment plan, please go to a regular hospital for treatment.
(the picture comes from the Internet. If there is infringement, please delete it by private letter)
